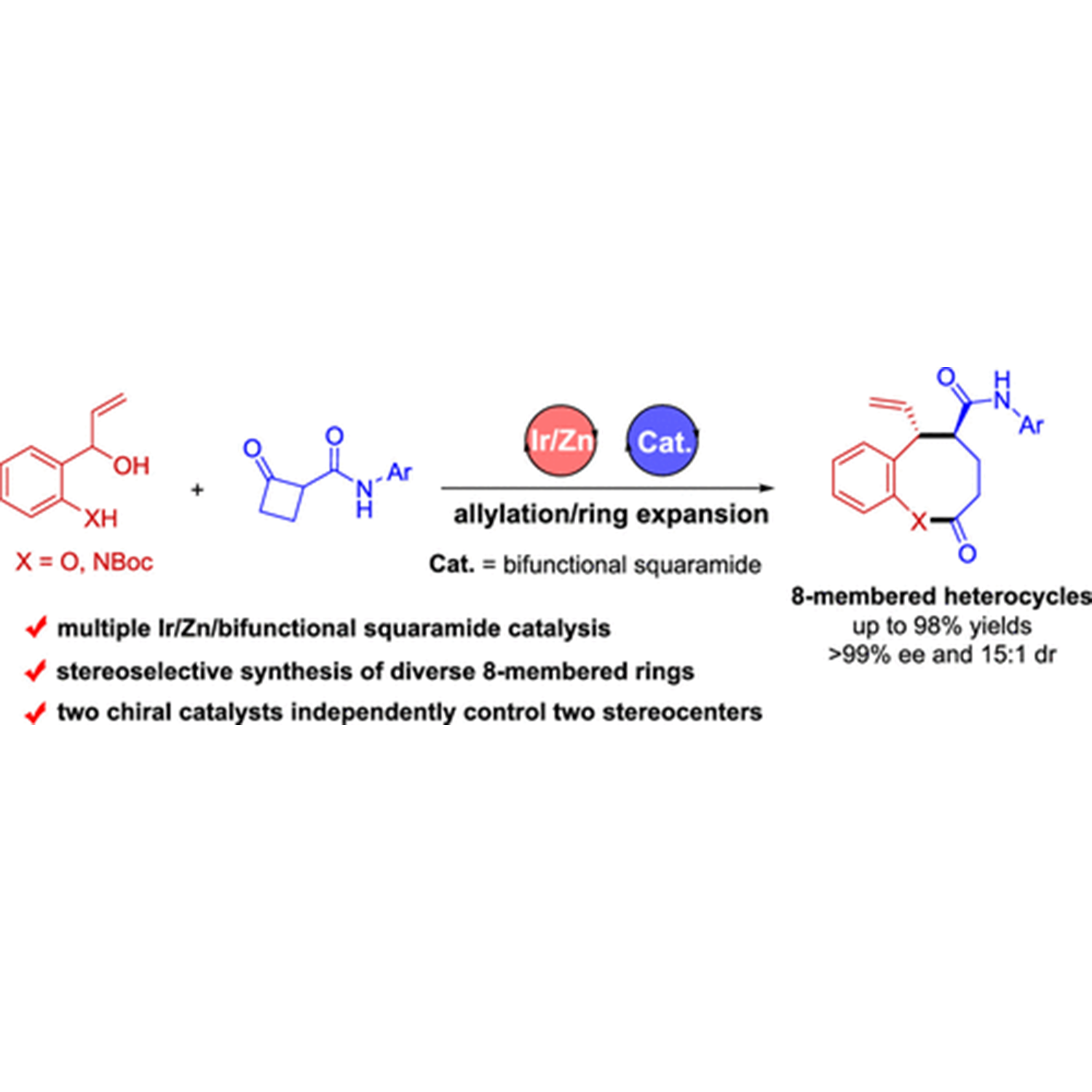
ABSTRACT: The development of protocols for constructing chiral medium-sized heterocycles with high efficiency and excellent stereocontrol is of great interest owing to their ubiquitous occurrence in natural products and biologically active pharmaceuticals. Nonetheless, current synthetic approaches are limited due to unfavorable enthalpy and entropy factors, as well as transannular interactions. The present work addresses this issue by designing an asymmetric allylation/ring expansion reaction of 2-(1-hydroxyallyl)phenols and cyclobutanone carboxamides enabled by sequential iridium/zinc/bifunctional squaramide catalysis, affording a series of 8-membered benzo[b]oxocines in high yields with high diastereo- and enantioselectivities. Mechanistic investigation reveals that the enantioselectivity is controlled by the chiral iridium catalyst, while density functional theory calculations demonstrate that the diastereoselectivity is controlled by the chiral bifunctional squaramide catalyst. Moreover, the sequential allylation reaction strategy is demonstrated to be also applicable to the synthesis of two types of enantiomerically enriched nitrogen heterocycles, 8-membered benzo[b]azocines and polycyclic cyclobuta[b]quinolines.
For detail:https://doi.org/10.1021/acscatal.1c03711