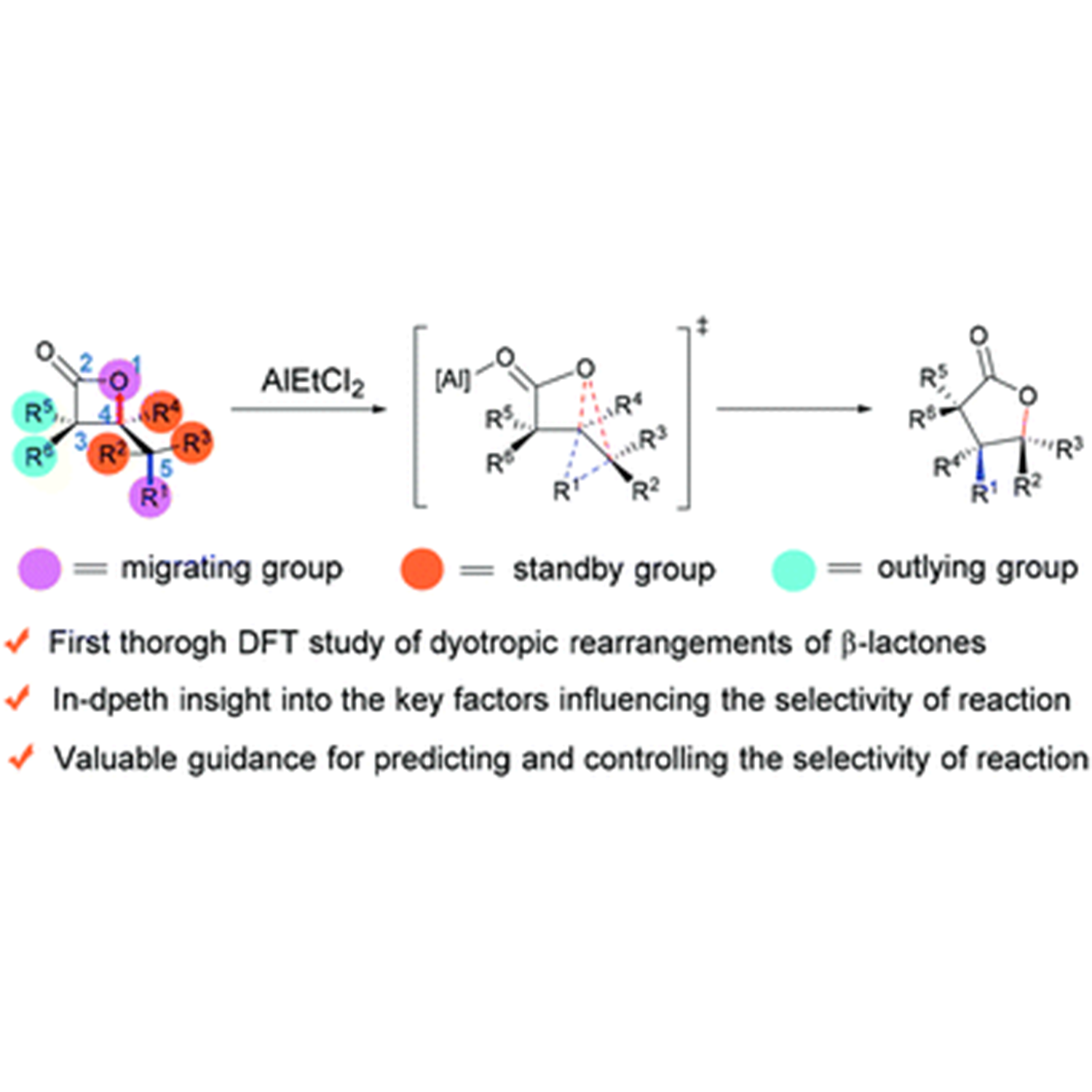
Organic Chemistry Frontiers | Elucidating the selectivity of dyotropic rearrangements of β-lactones: a computational survey
ABSTRACT: The dyotropic rearrangement of β-lactones is a neglected treasure in the family of multi-bond reactions and pericyclic reactions. Despite its appealing synthetic potential, the complicated migration selectivity greatly limits its widespread application. In this work, we report the first systematic and comprehensive computational study on the dyotropic rearrangements of β-lactones. The use of the double-hybrid functional ensures the accuracy of results. On the basis of the present study and our previous work, five methods to control the reaction selectivity of dyotropic rearrangements of β-lactones have been summarized, providing valuable references for synthetic chemists to design and develop brand-new type dyotropic reactions. For detail:https://doi.org/10.1039/D1QO01591E
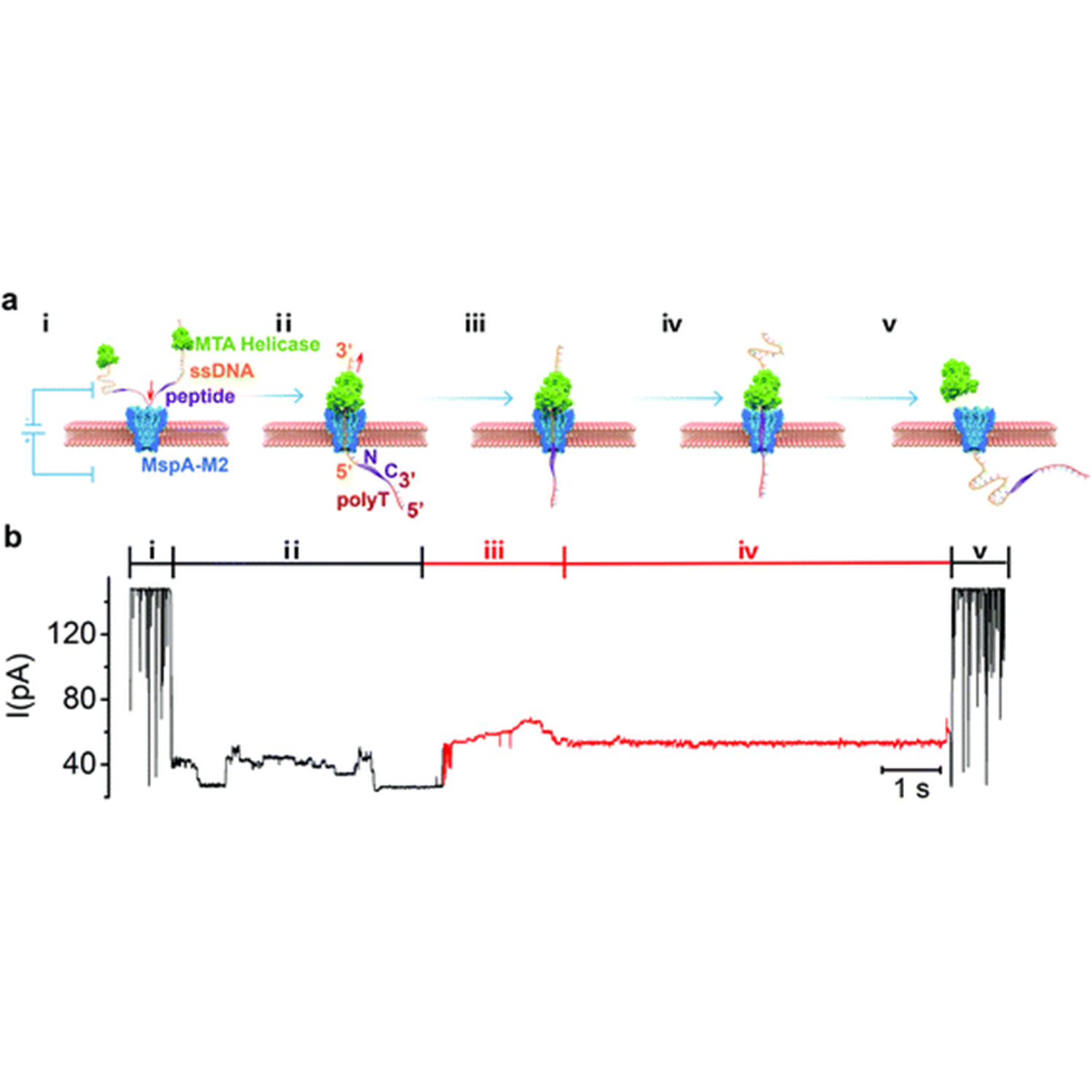
Chemical Science | Controlled movement of ssDNA conjugated peptide through Mycobacterium smegmatis porin A (MspA) nanopore by a helicase motor for peptide sequencing application
ABSTRACT: The lack of an efficient, low-cost sequencing method has long been a significant bottleneck in protein research and applications. In recent years, the nanopore platform has emerged as a fast and inexpensive method for single-molecule nucleic acid sequencing, but attempts to apply it to protein/peptide sequencing have resulted in limited success. Here we report a strategy to control peptide translocation through the MspA nanopore, which could serve as the first step toward strand peptide sequencing. By conjugating the target peptide to a helicase-regulated handle-ssDNA, we achieved a read length of up to 17 amino acids (aa) and demonstrated the feasibility of distinguishing between amino acid residues of different charges or between different phosphorylation sites. Further improvement of resolution may require engineering MspA-M2 to reduce its constriction zone's size and stretch the target peptide inside the nanopore to minimize random thermal motion. We believe that our method in this study can significantly accelerate the development and commercialization of nanopore-based peptide sequencing technologies. For detail:https://doi.org/10.1039/D1SC04342K
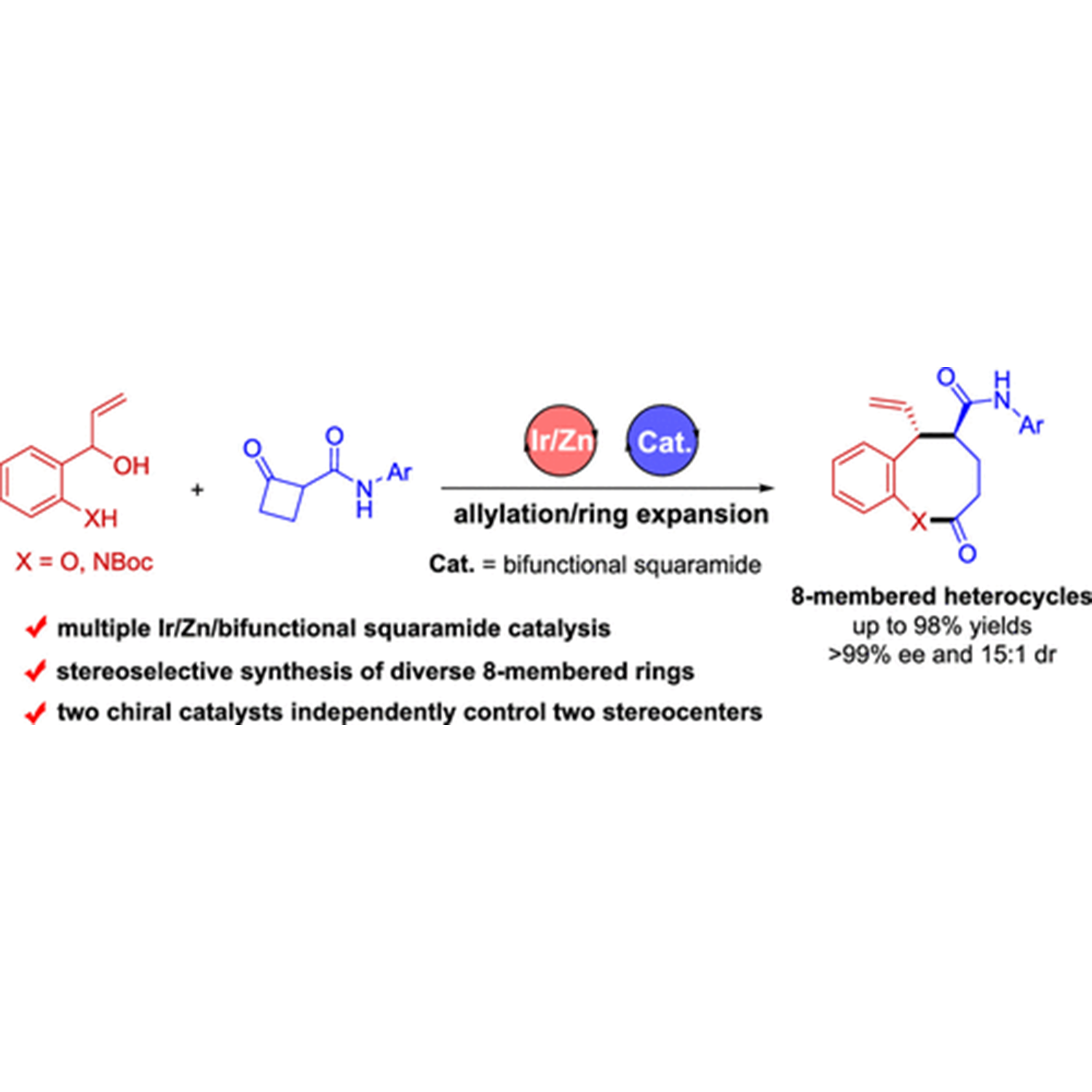
ACS Catalysis | Diastereo- and Enantioselective Synthesis of Eight-Membered Heterocycles via an Allylation/Ring Expansion Sequence Enabled by Multiple Catalysis
ABSTRACT: The development of protocols for constructing chiral medium-sized heterocycles with high efficiency and excellent stereocontrol is of great interest owing to their ubiquitous occurrence in natural products and biologically active pharmaceuticals. Nonetheless, current synthetic approaches are limited due to unfavorable enthalpy and entropy factors, as well as transannular interactions. The present work addresses this issue by designing an asymmetric allylation/ring expansion reaction of 2-(1-hydroxyallyl)phenols and cyclobutanone carboxamides enabled by sequential iridium/zinc/bifunctional squaramide catalysis, affording a series of 8-membered benzo[b]oxocines in high yields with high diastereo- and enantioselectivities. Mechanistic investigation reveals that the enantioselectivity is controlled by the chiral iridium catalyst, while density functional theory calculations demonstrate that the diastereoselectivity is controlled by the chiral bifunctional squaramide catalyst. Moreover, the sequential allylation reaction strategy is demonstrated to be also applicable to the synthesis of two types of enantiomerically enriched nitrogen heterocycles, 8-membered benzo[b]azocines and polycyclic cyclobuta[b]quinolines. For detail:https://doi.org/10.1021/acscatal.1c03711
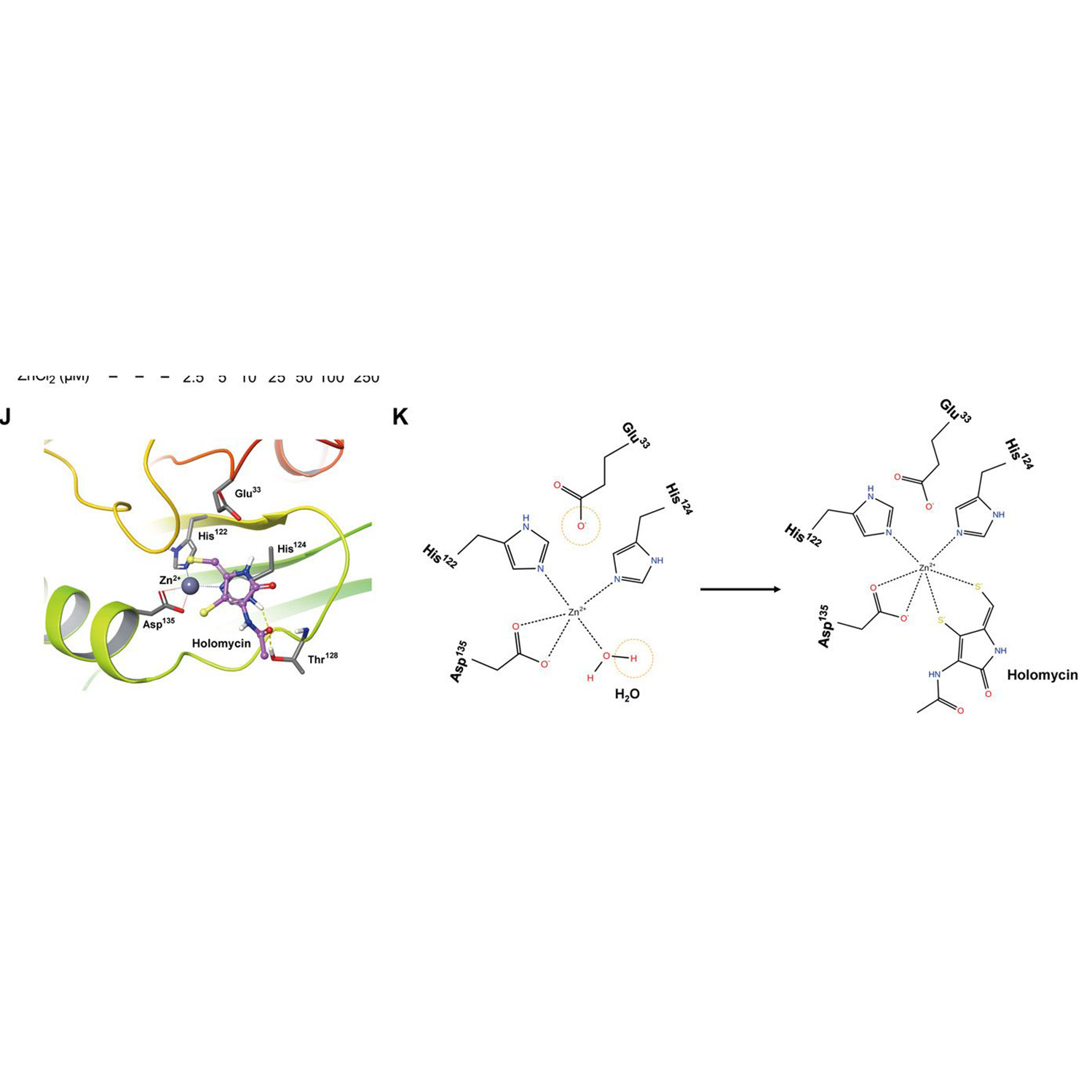
Science Immunology | Pharmacological targeting of NLRP3 deubiquitination for treatment of NLRP3-associated inflammatory diseases
ABSTRACT: Pharmacologically inhibiting nucleotide-binding domain and leucine-rich repeat-containing (NLR) family, pyrin domain–containing protein 3 (NLRP3) inflammasome activation results in potent therapeutic effects in a wide variety of preclinical inflammatory disease models. NLRP3 deubiquitination is essential for efficient NLRP3 inflammasome activity, but it remains unclear whether this process can be harnessed for therapeutic benefit. Here, we show that thiolutin (THL), an inhibitor of the JAB1/MPN/Mov34 (JAMM) domain–containing metalloprotease, blocks NLRP3 inflammasome activation by canonical, noncanonical, alternative, and transcription-independent pathways at nanomolar concentrations. In addition, THL potently inhibited the activation of multiple NLRP3 mutants linked with cryopyrin-associated periodic syndromes (CAPS). Treatment with THL alleviated NLRP3-related diseases in mouse models of lipopolysaccharide-induced sepsis, monosodium urate–induced peritonitis, experimental autoimmune encephalomyelitis, CAPS, and methionine-choline–deficient diet-induced nonalcoholic fatty liver disease. Mechanistic studies revealed that THL inhibits the BRCC3-containing isopeptidase complex (BRISC)–mediated NLRP3 deubiquitination and activation. In addition, we show that holomycin, a natural methyl derivative of THL, displays an even higher inhibitory activity against NLRP3 inflammasome than THL. Our study validates that posttranslational modification of NLRP3 can be pharmacologically targeted to…
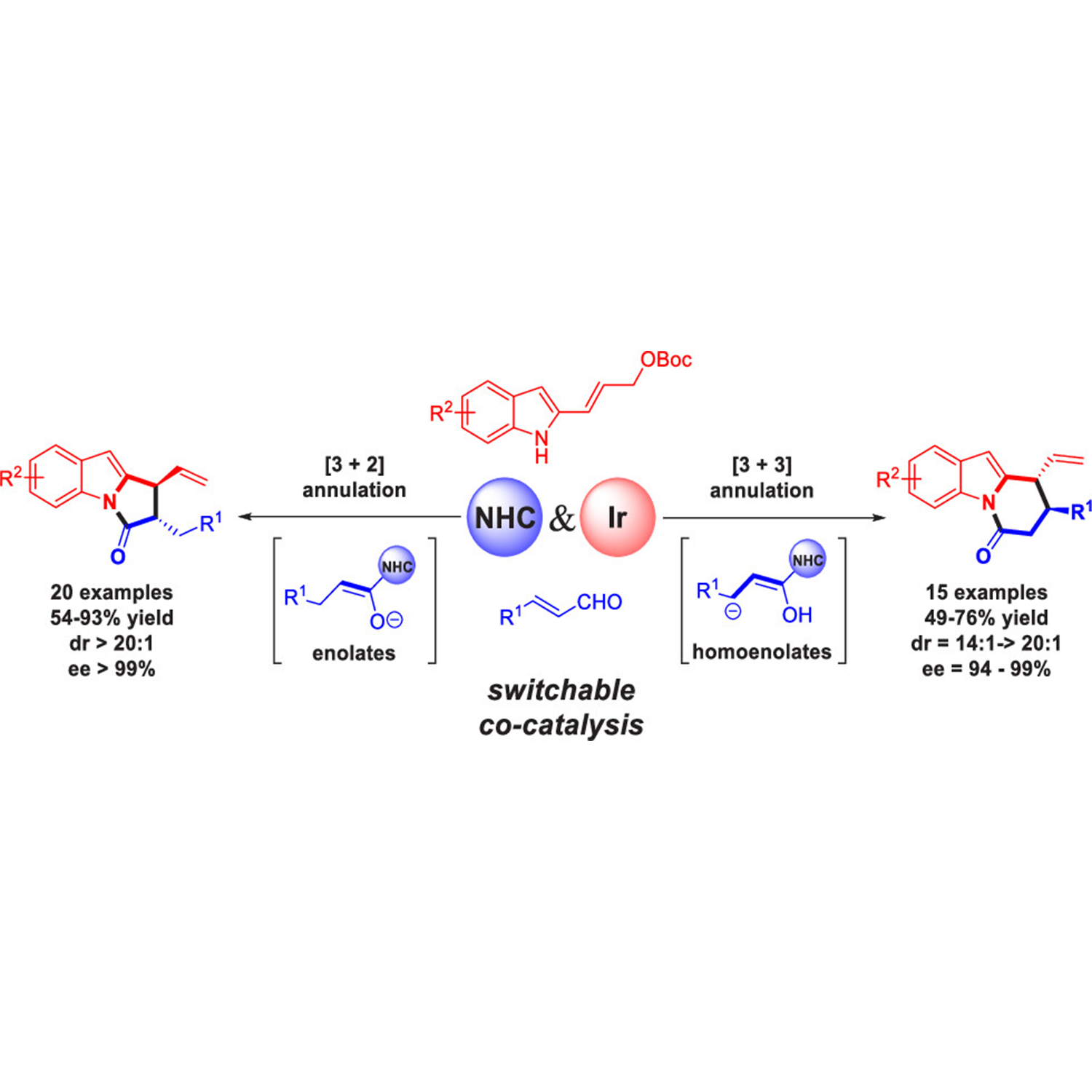
ACS Catalysis | Cooperative N-heterocyclic Carbene and Iridium Catalysis Enables Stereoselective and Regiodivergent [3 + 2] and [3 + 3] Annulation Reactions
ABSTRACT: A cooperative N-heterocyclic carbene (NHC)/iridium catalysis has been developed to achieve highly stereoselective and regiodivergent [3 + 2] and [3 + 3] annulation reactions of 2-indolyl allyl carbonates with enals. The use of the NHC catalyst has introduced switchable homoenolate and enolate intermediates from the common enal precursor via a simple adjustment of reaction conditions in a predictable manner. This protocol furnishes two types of biologically important products, pyrrolo[1,2-a]indoles and pyridine[1,2-a]indoles, with high diastereo- and enantioselectivities (up to >20:1 dr and >99% ee). Notably, all four stereoisomers of these products with two vicinal stereocenters could be afforded through permutations of the enantiomers of the two chiral catalysts. Mechanistic investigations and further computational density functional theory calculations give an explanation of the origin of the regioselectivity. In addition, the NHC-enolate intermediate generated from formylcyclopropanes was also compatible in this cooperative catalytic system and thus the arsenal of optically pure pyrrolo[1,2-a]indole products was enriched. For detail:https://pubs.acs.org/doi/abs/10.1021/acscatal.1c00081